You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
What is now considered an epidemic by some, the methamphetamine crisis in America has reached an all-time high, with more than 102,000 methamphetamine-related emergency department (ED) visits occurring in 2011 alone.1 The number of these visits has increased exceedingly since 1992, with the criminal justice system referring many of them.2 The RAND Corporation has estimated the annual economic burden methamphetamine use places on society, including treatment, healthcare costs, disruption of family life, loss of productivity, criminal costs, and death, to be $23.4 billion.3
Methamphetamine use has a long history.2,4,5 The crystallized form—crystal meth—provides a drug that is relatively easy to produce, inexpensive to purchase, and longer lasting than its opioid counterparts. This washed, crystallized version of methamphetamine has a purity of up to 98%.6 Because crystal meth can be injected, swallowed, or smoked, is easy to transport, and is said to result in more intense “trips” that can last up to 12 hours, it is the drug of choice among methamphetamine users. Discontinuing its use is extremely difficult due to the withdrawal symptoms of anxiety, irritability, restlessness, fatigue, and dysphoria,7 forcing users to partake in what is known as “tweaking.” “Tweakers” reinforce their “high” by using the drug for periods of time ranging from days to weeks, allowing themselves to defer facing withdrawal. The eventual result is a terrible withdrawal consisting of a restless, sometimes unarousable, sleep with sweats followed by sores, itching, decayed teeth, and nightmarish feelings of bugs crawling on their skin.
Long-term users of crystal meth have said that they are unable to remember large periods of their life, even forgetting where they once lived. Some have long-lasting psychiatric problems and need to take such drugs as Prozac every day just to feel better. Chronic abuse can cause psychotic and violent behavior characterized by intense paranoia, visual and auditory hallucinations, and out-of-control rages, even in those who have been abstinent for years.6
In light of concerns regarding the drug’s ability to cause long-term neurological damage and cognitive impairment, numerous studies have been conducted that have researched the effects of methamphetamines. Some studies have looked at the biochemical effects of methamphetamines, while others have examined differences in brain mass and volumes in users compared to non-users.5 In addition to discussing the public health concern caused by methamphetamine use, this article will describe the major physiologic and psychological effects of meth as noted in both animal and human studies and will examine the neurotoxic effects that meth has on serotonin and dopamine neurons in the brain. It will describe the factors involved in the neurophysiology of meth and discuss factors relating to its strong addictive and neurotoxic potential. Additionally, it will present ways in which dentists can treat this patient population.
Public Health Concern
The use of methamphetamines is a public health concern not only in America but the entire world, with more than 35 million people regularly using meth worldwide.8 The United States is seventh on the list of the highest prevalence of amphetamine-type stimulant users, most of whom use meth.9 Moreover, the epidemic is spreading; while the numbers of users have begun to stabilize in the western US states, the eastern US states are just starting to experience the problem.
With increased use comes a strong burden on the population at large and state governments. As discussed above, longer-term use can result in substantial cognitive, social, and psychological deficits as well as neurotoxicity. Though numerous studies have examined the effects of meth in these areas, treatments for these conditions have, at best, had a minimal impact. Those who abuse meth are also at risk of sexually transmitted and bloodborne diseases as they become compulsive and obsessive with sexual behaviors.9-11 There is also an increased risk of other health and social consequences.12 As Hawaii and California have experienced, patients who were positive for meth were more likely to have intentional self-inflicted injuries, suicide,10 or intentional assaults and were also more likely to end up in prison.9
The National Drug Intelligence Center has even put out a warning about the volatility of the vapors used in the manufacturing of the drug as the number of burn patients from meth lab explosions has increased. Consequently, the number of patients admitted to burn centers for severe burns has also risen, with a mean cost to the hospital of $77,580 per patient as meth users rarely have the means to pay for their treatment.9 Additionally, children (including those exposed in utero) exposed to these environments (ie, at-home meth labs) have been found to tend to have developmental delays.13
Another issue associated with meth production is waste disposal. State governments have a difficult time disposing of the waste, as one lab can manufacture up to 10 pounds of meth in a 24-hour period and each pound of meth creates six pounds of hazardous waste that must be disposed.8 This is a significant economic burden and a problem that needs to be addressed because research alone into the effects of meth cannot change the situation.
Physiology of Meth
Meth is an indirectly acting sympathomimetic that precipitates a massive, sustained release of dopamine in the brain, causing the “high” that users pursue.14 Meth’s activity differs as a function of location in the brain. There are three major dopaminergic systems: the nigrostriatal, mesolimbic, and mesocortical systems. In the striatum, meth stimulates the release of dopamine via five mechanisms. It increases the release of dopamine from storage vesicles; inhibits monoamine oxidase, the enzyme responsible for breaking down dopamine; blocks the reuptake at the synaptic cleft; decreases the expression of dopamine transporters at the cell surfaces; and increases the expression of tyrosine hydroxylase (which allows the conversion of tyrosine into dopamine).8
In the nigrostriatal pathway, the released dopamine stimulates the D1 receptors on the gamma amino butyric acid (GABA) neurons causing an increased release of GABA, the main inhibitory neurotransmitter in the brain. GABA then stimulates the GABA (a) receptors on the postsynaptic neurons, resulting in the inhibition of the nigrothalamic projections. Because these projections are also inhibitory, their silence consequently allows the stimulation of the thalamocortical projections. These projections then increase their release of glutamate, the major stimulatory neurotransmitter in the brain.15 Glutamate stimulates the striatum and, together with the increased release of dopamine, can lead to oxidative and excitatory neurotoxic damage15 as well as the positive symptoms in meth addicts that are similar to those seen in schizophrenics (euphoria, hallucinations, strange or unusual beliefs and alertness, increased libido, and decreased appetite8,9).
As a central acting stimulant, meth exerts its effect on almost every organ, from the heart and adrenal glands to the lungs and kidneys. Briefly, meth can cause chest pain, hypertension, and myocardial infarctions, as well as dyspnea and wheezing. Its most severe effects are strokes, pulmonary edema, necrotizing angiitis with arterial aneurysms, and sacculations in the kidney, liver, and pancreas. Notably, meth (and also Ecstasy) reduces both salivary quality and quantity, resulting in xerostomia and Sicca syndrome; this is due possibly to stimulation in the brain of alpha2-sympathetic adrenoreceptors, inhibiting salivary secretion.16
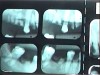
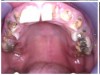
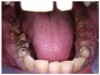
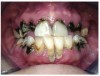
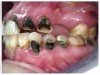
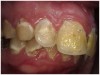
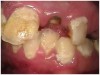
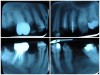
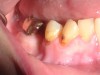
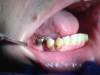
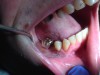
Often, the most visually striking manifestation of long-term meth use—known as “meth mouth”—is caused by the lack of saliva combined with an increase in soda drinking and extremely poor oral hygiene. This typically leads to meth abusers experiencing a large amount of caries. Decay begins with occlusal and facial caries and progresses rapidly, decaying to the bone level and often leaving only roots (Figure 1 through Figure 3).16
Examples of Oral Destruction
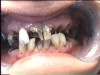
The patient shown in Figure 4 through Figure 6 was a meth user for 6 years. He had a stroke at age 22. After recovering from the stroke and stabilization of his circulatory system was achieved, and once he quit using meth, he strongly desired to apply for a job and wished to improve his dental appearance. He opted to have all of his teeth extracted, as complete dentures were his only treatment option because there were no adequate abutment teeth remaining for placement of removable partial dentures.
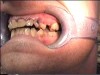
In Figure 7 through Figure 9, the young woman shown had many adverse childhood experiences and was given methamphetamine by an adult when she was 14 years old. She also had her first child at that age, and then had three more children while addicted to meth, though she was only able to retain custody of one of her children. In addition to alcohol, she used meth for 15 years. Her only prosthetic option was extraction of all of her remaining teeth and subsequent placement of complete dentures.
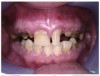
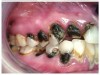
The patient in Figure 10 through Figure 12 was pregnant when she presented for dental treatment. The pregnancy was unplanned and she had had a lack of prenatal care. Continuing meth use during the pregnancy resulted in a positive toxic baby. She stated that she had just stopped using meth and had entered a rehabilitation facility a few days before. She was brought to the author’s clinic with a toothache. Note that as seen in these images, extreme accumulation of plaque, lack of oral hygiene, and severely decayed and missing teeth are classic manifestations in meth users.
What Dentists Can Do to Help
Caring for patients who abuse methamphetamine is challenging. When meth use is ongoing it is extremely difficult to mitigate the dentally destructive effects of this substance. Early interventions will have the best outcomes in terms of saving teeth. Because of the intense xerostomia associated with methamphetamine, patients who use it are automatically classified as “extreme caries risk.”17 The authors propose a dual-purpose approach: caries arrest combined with oral hygiene improvement and prevention. The former may be accomplished with the use of silver diamine fluoride (SDF).
SDF is an antimicrobial liquid and desensitizing agent used to treat dentin hypersensitivity. It is available in the United States as a 38% solution. Like other desensitizing agents being used off-label as best practice for preventing incident dental caries, SDF offers the additional promise of arresting extant dental caries.18-20 SDF accomplishes this by being bactericidal and forming an acid-resistant protective layer on the tooth. The silver in SDF kills the cariogenic biofilm bacteria, and the fluoride inhibits demineralization and promotes remineralization of the dental hard tissue by converting hydroxyapatite to fluorapatite.19,20 As a result of applying SDF to a tooth surface, black staining precipitates as silver salts (silver phosphate), which become embedded in the active decay lesion, adjacent biofilm, and/or soft tissue. This light-sensitive compound will react to turn the decay from soft and brown to hard and black, and it may then be restored definitively as needed. The staining effect on the biofilm and soft tissue is transient and may be polished away or allowed to naturally desquamate (Figure 13 through Figure 15). Healthy tooth structure will remain unchanged and will not stain black.
Dental professionals are currently using SDF to help slow or stop the caries process on accessible tooth surfaces until the patient is able to be safely treated with conventional dental care to restore esthetics and function. SDF protocol is a promising, evidence-informed, science-based practice that is particularly helpful for a variety of situations.17 These include very young children who cannot be treated in a traditional dental office setting and may be waiting for a hospital dental appointment; patients with extreme caries risk due to xerostomia; patients with complex behavioral or medical comorbidities that make conventional dental treatment difficult; patients with carious lesions affecting the entire permanent dentition, making it unfeasible to treat in a timely manner; carious lesions on surfaces that are difficult to treat clinically due to accessibility; and use in population-based low-intensity dental disease prevention strategies by street outreach workers in lieu of access to dental care.21 SDF can also be used to safely delay extensive treatment to allow for primary prevention practices to be adopted or to allow time for other, more urgent life circumstances to be addressed such as substance misuse, rehabilitation, or homelessness.
Effective prevention counseling and treatment with current meth users or methadone maintenance therapy (MMT) clients requires use of an interprofessional model of healthcare delivery. Prevention requires a holistic approach and competent communication.22 Holistic prevention takes into consideration the related health and economic issues MMT clients face, such as poor self-esteem, depression, and often lower socioeconomic status. MMT clients tend to also present undernourished and with anxiety disorders, diabetes, asthma, and poor general health.23
People in recovery, ie, current methamphetamine users or MMT patients, exhibit similar characteristics when presenting to health professionals. Often these individuals present with behavioral and psychological challenges that create barriers to following prevention recommendations and accessing oral healthcare.24 Charnock et al showed that while almost 60% of nondrug users made use of dental services regularly, only 29% of drug users did so.25
Specific prevention services, health counseling, and product recommendations by dental professionals are based upon the risk assessment.
Xerostomia and high caries risk are common debilitating oral findings of meth users and MMT clients. A primary prevention goal is to establish and maintain a neutral pH of the oral cavity. Use of a baking soda (sodium bicarbonate) water rinse is an economical, readily available method to achieve a non-acidic oral environment. To stimulate saliva, sugar-free chewing gum containing xylitol can be used.
The cost and availability of prevention products to establish a neutral oral pH and enhance enamel remineralization varies. Examples of prevention products are PreviDent® (Colgate Professional, www.colgateprofessional.com), Fluoride Varnish™ (3M ESPE, www.3m.com), MI Paste™ (GC America Inc., www.mi-paste.com), and CTx4 Treatment Rinse (CariFree, www.carifree.com). For direct preventive dental services, a reduced length of appointment time of 20 to 30 minutes for a dental cleaning and avoiding deep scaling during recovery withdrawal periods have been recommended.24 In addition to brushing three times daily and flossing twice daily, a variety of products may be implemented immediately for patients with methamphetamine-associated xerostomia. Some products include ACT® Dry Mouth mouthwash, lozenges, and toothpaste (Chattem Inc., www.actoralcare.com), Xerostom® (Biocosmetics Laboratories, www.biocosmetics.es), Xylitol gum (Epic Dental LLC, www.epicdental.com), and Dry Mouth Relief (Colgate Professional).
The essential goal of an integrated team of health professionals for a meth user or MMT client is to encourage recovery, improve re-socialization, decrease dental anxiety, and restore general and oral health. Hygiene improvement and preventive dentistry utilizes a caries risk assessment. Patients using methamphetamines fall into the extreme risk category and may be cared for by adopting protocols appropriate for that group. Table 1 outlines caries risk protocols for patients in this category.26
Working Together
The interprofessional team model is essential to address prevention and oral health for meth users and MMT clients. To counteract the potential cariogenic effect of the sucrose syrup, methadone can be prepared in a sugar-free solution.27 Practical prevention counseling and preventive services delivered by nurses, physicians, nutritionists, and behavioral and social service professionals complement the services of dentists, dental therapists, and dental hygienists.
ABOUT THE AUTHORS
Lola Giusti, DDS, MA
Assistant Professor, Department of Dental Practice, University of the Pacific, Dugoni School of Dentistry, San Francisco, California; Private Practice, San Francisco, California
Jamie Jenkins MD, RDMS
Education Director, St. Clare Hospital, Lakewood, Washington; TeamHealth, Emergency Medicine Attending Physician
Mitchell A. Goodis, DDS
Private Practice, El Dorado, California
Carsen Bentley, DDS, MPH
Instructor, Department of Dental Practice and Community Programs, University of the Pacific, Dugoni School of Dentistry, San Francisco, California
Christine E. Miller, MPH, RDH
Associate Professor, Director of Community Health Programs, Department of Dental Practice and Community Programs, University of the Pacific, Dugoni School of Dentistry, San Francisco, California
Alexander Faigen, DMD
Oral Surgery Resident, University of Pittsburgh School of Dentistry, Pittsburgh, Pennsylvania
REFERENCES
1. Emergency Department Visits Involving Methamphetamine: 2007-2011. Substance Abuse and Mental Health Services Administration (SAMHSA) website. June 19, 2014. www.samhsa.gov/data/sites/default/files/DAWN_SR167_EDVisitsMeth_06-12-14/DAWN-SR167-EDVisitsMeth-2014.htm. Accessed May 1, 2017.
2. Lineberry TW, Bostwick JM. Methamphetamine abuse: a perfect storm of complications. Mayo Clin Proc. 2006;81(1):77-84.
3. Nicosia N, Pacula RL, Kilmer B, et al. The Economic Cost of Methamphetamine Use in the United States, 2005. Santa Monica, CA: RAND Corporation, Drug Policy Research Center; 2009.
4. eMedicine Clinical Knowledge Base, Institutional Edition; Toxicity, Methamphetamine. www.emedicine.com.
5. Chang L, Cloak C, Patterson K, et al. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57(9):967-974.
6. National Drug Intelligence Center. Information Bulletin: Crystal Methamphetamine. August 2002. www.justice.gov/archive/ndic/pubs1/1837/index.htm. Accessed May 1, 2017.
7. Petit A, Karila L, Chalmin F, Lejoyeux M. Methamphetamine addiction: a review of the literature. J Addict Res Ther. 2012:S1:006. doi:10.4172/2155-6105.S1-006.
8. Barr AM, Panenka WJ, MacEwan GW, et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31(5):301-313.
9. Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18(3):235-242.
10. Chana G, Everall IP, Crews L, et al. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67(8):1486-1489.
11. Worth H, Rawstorne P. Crystallizing the HIV epidemic: methamphetamine, unsafe sex, and gay diseases of the will. Arch Sex Behav. 2005;34(5):483-486.
12. Callor WB, Petersen E, Gray D, et al. Preliminary findings on noncompliance with psychotropic medication and prevalence of methamphetamine intoxication associated with suicide completion. Crisis. 2005;26(2):78-84.
13. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57(2):255-260.
14. Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54(6);1344-1349.
15. Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24(50):11449-11456.
16. Shaner JW, Kimmes N, Saini T, Edwards P. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDS. 2006;20(3):146-150.
17. Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications, and consent. J Calif Dent Assoc. 2016;44(1):16-28.
18. Llodra JC, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84(8):721-724.
19. Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet.” J Dent Res. 2009;88(2):116-125.
20. Featherstone JDB, Horst JA. Fresh approach to caries arrest in adults. Decisions in Dentistry. 2015;1(1):36-44.
21. Brown C, Krishnan S, Hursh K, et al. Dental disease prevalence among methamphetamine and heroin users in an urban setting: a pilot study. J Am Dent Assoc. 2012;143(9):992-1001.
22. Robinson PG, Acquah S, Gibson B. Drug users: oral health-related attitudes and behaviours. Br Dent J. 2005;198(4):219-224.
23. Titsas A, Ferguson MM. Impact of opioid use on dentistry. Aust Dent J. 2002:47(2):94-98.
24. Brondani M, Park PE. Methadone and oral health—a brief review. J Dent Hyg. 2011:85(2):92-98.
25. Charnock S, Owen S, Brookes V, Williams M. A community based programme to improve access to dental services for drug users. Br Dent J. 2004;196(7):385-388.
26. Jenson L, Budenz AW, Featherstone JD, et al. Clinical protocols for caries management by risk assessment. J Calif Dent Assoc. 2007;35(10):714-723.
27. Sheedy JJ. Methodone and caries. Case reports. Aust Dent J. 1996;41(6):367-369.