You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The competitive nature of the dental-implant industry has increased awareness of alternative dental implants, such as high-strength ceramics (eg, zirconium oxide/zirconia). Due to its more esthetic and biocompatible properties, zirconia has better soft-tissue response and more closely mimics natural tooth structure. Optimizing periodontal health and minimizing potential for peri-implantitis, zirconia provides less bacterial accumulation around the ceramic implant and bone-soft-tissue assembly. Ceramic dental implants provide an alternative to metal implants for long-term replacement of missing teeth and their restoration. Dental patients now have the opportunity to select a material of their choice for dental implantology and long-term restorations. Through case studies, this article will discuss the workflow in restoration with ceramic dental implants, using traditional crown-and-bridge techniques for developing soft tissue and emergence profiles for the restorations. The article also will relate similarities between restoration with ceramic dental implants and natural dentition.
Case One: Placement and Splinting of Multiple Ceramic Implants
A patient presented to the dental practice with multiple root fractures, recurrent caries, and a need for significant crown-lengthening procedures, endodontic therapy, post and cores, and long-term restorations. The patient considered the entire volume of therapy and was not interested in undergoing treatment with less than a satisfactory long-term prognosis. The patient was given two dental-implant options and chose to move forward with ceramic dental implants.
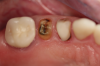
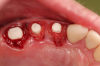
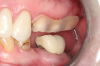
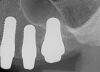
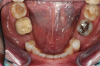
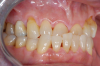
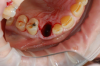
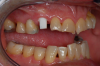
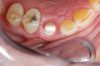
Figure 1 and Figure 2 depict the preoperative image and subsequent removal of three posterior teeth, immediate placement of zirconia-oxide ceramic dental implants, and soft-tissue augmentation with platelet-rich fibrin (PRF) to enhance the soft-tissue architecture surrounding the ceramic implants. The teeth were removed atraumatically and without suture placement. Because the implants were one-piece in stature (the abutment was incorporated into the implant), the recommendation was to splint the multiple units to minimize the lateral forces from mastication, swallowing, and tongue movement.
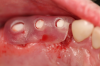
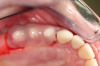
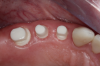
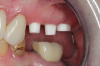
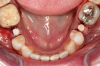
Figure 3 through Figure 5 show the fitting of a one-piece multiple splint using a light-cured acrylic, along with flowable composite to adhere the splint to the three ceramic dental implants. The splint was to remain out of occlusion, without any occlusal forces for the 12-week osseointegration period. The patient was instructed to chew on the other side of her mouth for the 12 weeks before returning to the dental practice for simple removal of the splint. Removal of the splint revealed optimum results. The soft tissues were pink, with keratinized gingival margins completely surrounding the ceramic implants and consistent with what most dentists observe with the periodontium around periodontally healthy natural teeth (Figure 6 and Figure 7).
In some instances after the osseointegration periods, the soft tissue migrates occlusally due to the biocompatibility of ceramic implants. Now restorative dentists have the option of retraction chord, CO2 laser gingivectomy, or diode-laser gingivectomy to expose the margins. If the margins are supragingival, a high-speed zirconia cutting bur can be used to create a finish margin for the long-term restoration.
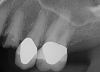
Essentially, after integration and evaluation of soft tissue at 12 to 16 weeks subsequent to surgical placement of the ceramic implant, the implant is treated similarly to a natural tooth receiving a long-term restoration, and the protocol is the doctor's choice for soft-tissue management at the time of the final impression. Radiographic examination in Figure 8 and Figure 9 revealed that bone volume and soft tissue were preserved, primarily due to exceptional biocompatibility and the PRF biologics implemented after the atraumatic extractions.
Case Two: Restoring Natural Dentition and Placing Ceramic Dental Implants
Often a dentist will evaluate, diagnose, treatment-plan and deliver a case presentation to a patient who requires multiple restorations to restore natural dentition and missing teeth. After the patient understands and accepts the treatment plan provided by the dentist, the workflow to begin restoring natural dentition and placing ceramic dental implants is developed using a diagnostic wax up and provisionals that mimic natural dentition. Provisionals are used to help develop soft-tissue architecture, provide light loading forces to the surrounding bone and soft tissues after the initial osseointegration, and give the dentist positive or negative feedback from the patient regarding food impaction or occlusal disharmonies.
Figure 10 through Figure 13 show the preoperative x-ray and the sequence of beginning with healed sites through the surgical placement of the implants. The implants were protected by an Essix-style wound-protection removable retainer for approximately 12 weeks. After the integration phase, the implants and the natural dentition were prepared using traditional crown-and-bridge high-speed diamond and zirconia cutting burs to remove decay and existing restorative materials, to complete and refine the natural-tooth structures to receive full-crown coverage, and to prepare and refine gingival margins of the zirconia implants where needed.
After the preparations were evaluated and accepted by the clinician, provisionals were completed with traditional crown-and-bridge techniques. Then the provisionals were fabricated with a replication stent derived from the diagnostic wax up (Figure 14). The provisionals were well-fitted to both the natural dentition and the ceramic dental implants (Figure 15). The provisional that was fixed to the implant was kept out of occlusion and had a narrow buccal-lingual occlusal table. The smaller occlusal table and short occlusal scheme provided light loading forces and stimulated the bone tissue—the brain detects the stimuli and sends osteoblasts to the area to increase bone density surrounding the implant. The recommendation for the light-loading provisional phase was to have the patient function with the provisionals for 4 weeks.
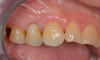
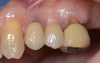
The final impression appointment follows the light-load phase, provided the implant is stable and the soft tissue is acceptable to the clinician. Polyvinyl putty and light-body impression materials were used in this case; analog casts and final full-zirconia full crowns were fabricated (Figure 16). The patient provided feedback that chewing was comfortable and without food impactions. Keratinized gingival cuff was developed, and natural-tooth contours for the restorations mimicked what is normally found in human dentition. The healed bone surrounding the ceramic dental implant was similar in density and crestal levels in comparison with the adjacent natural teeth, evident in the radiographic evaluation (Figure 17). Final long-term restorations at the gingival crest and restoration interfaces were also consistent in optimum periodontal health and gingival height. Arch-form integrity was upheld and occlusion was managed; osseointegration and light-load principles optimized the success of the restorations, and an acceptable, harmonious esthetic outcome was achieved for the patient.
Case Three: Maxillary-Anterior Ceramic-Implant Restoration and Provisional
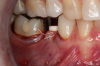
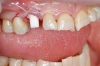
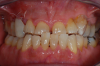
Occasionally dentists are presented with extreme challenges with long-term restorations and replacing missing teeth. Dentists must evaluate the patient's condition, develop optimum long-term oral health, and attempt to meet the patient's expectations. Figure 18 showed a class III malocclusion with deep bite, multiple abfractions, and less-than-ideal occlusal plane, but the patient wanted to replace his maxillary right canine only. A discussion with the patient regarding occlusal disharmonies and comprehensive recommendations for full-mouth rehabilitation revealed that the patient had a limited budget for dental care. The patient understood the ramifications of his comprehensive dental needs not being affordable. Decisions were made to atraumatically remove the maxillary right canine (Figure 19) and immediately place a ceramic dental implant (Figure 20). Polytetrafluoroethylene (PTFE) 4-0 sutures were placed to help support soft tissues, and then a full-arch Essix-style retainer with a flowable composite facial veneer was used to provide some esthetics during the patient's healing phase (Figure 21). The implant and the retainer were not to be in contact—the retainer may occlude with the opposing dentition and also be passive in relation to the ceramic implant.
Sutures were removed 2 weeks after surgical implant placement, and the patient was instructed to continuously wear his Essix-style retainer for 12 weeks. The retainer was worn while eating during the 12-week period. The patient returned at 12 weeks for soft-tissue and implant-bone evaluation (Figure 22). At this appointment, minimal lingual soft-tissue manipulation was performed by gingival abrasion to the keratinized soft tissue and an aluminum chloride gel was placed.
Figure 23 showed an example of gingival soft tissue creeping occlusally and over the lingual gingival margin of the one-piece ceramic dental implant. This supragingival migration was caused by the exceptional biocompatibility due to the acid-etched surface of the implant, a one-piece implant that prevents bacterial colonization that one may find with two-piece metal-implant systems.
The final impressions were completed, and a full ceramic crown was cemented. From the time of impression completion through the workflow in fabrication of the final long-term ceramic crown, all restorative steps can be accomplished with traditional crown-and-bridge techniques or digital techniques. An 18-month postoperative follow-up revealed healthy keratinized gingival tissue, the papillae were maintained, and bone volume was saved (Figure 24 and Figure 25).
Human Histology Study
A human histology study was undertaken at the University of Minnesota to assess soft- and hard-tissue attachment around zirconia implants. Three months after surgery, implant sites were trephined, removed from the mouth, and analyzed under a microscope. The histology slides were evaluated to determine the amount of direct bone contact to the ceramic implant surface. Results showed a considerable amount of bone-to-implant contact.
While bone contact was evaluated, additional promising histology in regard to soft tissue was revealed. The soft tissue had attached to the surface of the zirconia. Such soft-tissue attachment is rarely, if ever, observed with two-piece titanium implants because the connection of those implants is at bone level, where there are higher levels of bacteria that may lead to chronic inflammation, peri-implantitis, and the potential for implant integration failure. High-strength ceramics such as zirconia are increasingly being considered as alternatives to metal-ceramic restorations and implants. Zirconia is a more esthetic and biocompatible material than titanium. Zirconia has been found to exhibit a better soft-tissue response because of its nonconductive, noncorrosive surface, which is more resistant to bacterial accumulation.
The study was performed to evaluate the type and quality of bone and soft-tissue attachment on the surface of a zirconia implant through histologic analysis. Two patients underwent full-mouth oral rehabilitation and agreed to donate two of their implants for the analysis. Both patients were restored with fixed zirconia full-contour bridges 4 months after implant placement. The results demonstrated that the acid-etched surface of the one-piece zirconia implants appeared to achieve soft-tissue attachment and osseointegration 3 months after surgery, with virtually no sign of the inflammatory infiltrate that may be seen in two-piece titanium implants' bone-level prosthetic connections.
Figure 26 allows a visual inspection and comparison of the soft-tissue profiles of a two-piece titanium implant and neighboring one-piece ceramic implant. The image suggests that the ceramic material not only has a better attachment of soft tissue, but a better cosmetic component. The restoring and surgical dentists should consider thin soft-tissue profiles of the patient when deciding on the implant material of choice.
Conclusion
Patients are becoming savvier when it comes to alternative dental-implant materials and procedures. They are requesting non-metal alternatives. Clinical studies of ceramic implants with acid-etched surfaces have shown these implants to lead to less bone loss, improved soft-tissue architecture, and improved gingival health due to reduction of bacteria colonization at the implant platform-prosthetic connection interface. The restoration and final cementation of this style of implant originates at the gingival crest, so gingival pockets develop similarly to a natural tooth. Histologic analyses indicated that zirconia implants with acid-etched surfaces have the same amount of osseointegration capabilities as titanium implants. Patients with metal-free preferences for their overall health considerations, high sensitivities to some of the chemical elements found in titanium alloys, and higher esthetic values may benefit from ceramic implant materials because the materials appear to create an improved biologic assembly with fewer mechanical failures.
About the Author
Thomas M. Bilski, DDS
Private Practice
Independence, Ohio
Resources
1. Hartmann D, Letulé V, Schneider JJ, Flaig MJ. Metal implant sensitivity: clinical and histological presentation. Hautarzt. 2016; 67(5):373-379.
2. Ungersboeck A, Geret V, Pohler O, et al. Tissue reaction to bone plates made of pure titanium: a prospective, quantitative clinical study. J Mater Sci: Mater Med. 1995;6(4):223-229.
3. Lalor PA, Revell PA, Gray AB, et al. Sensitivity to titanium. A cause of implant failure? J Bone Joint Surg Br. 1991;73(1):25-28.
4. Gahlert M, Gudehus T, Eichhorn S, et al. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin Oral Implants Res. 2007;18(5):662-668.
5. Kohal RJ, Klaus G. A zirconia implant-crown system: a case report. Int J Periodontics Restorative Dent. 2004;24(2):147-153.
6. Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: a histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7(suppl 1):S13-S20.
7. Langhoff JD, Voelter K, Scharnweber D, et al. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: a study in sheep. Int J Oral Maxillofac Surg. 2008;37(12):1125-1132.
8. Franchi M, Orsini E, Trire A, et al. Osteogenesis and morphology of the peri-implant bone facing dental implants. ScientificWorldJournal. 2004;4:1083-1095.
9. Franchi M, Bacchelli B, Giavaresi G, et al. Influence of different implant surfaces on peri-implant osteogenesis: histomorphometric analysis in sheep. J Periodontol. 2007;78(5):879-888.
10. Sollazzo V, Pezzetti F, Scarano A, et al. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008;24(3):357-361.
11. Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993;69(6):599-604.
12. Depprich R, Ommerborn M, Zipprich H, et al. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008;4:29.
13. Wenz HJ, Bartsch J, Wolfart S, Kern M. Osseointegration and clinical success of zirconia dental implants: a systematic review. Int J Prosthodont. 2008;21(1):27-36.
14. Özkurt Z, Kazazoğlu E. Zirconia dental implants: a literature review. J Oral Implantol. 2011;37(3):367-376.
15. Sghaireen MG. Fracture resistance and mode of failure of ceramic versus titanium implant abutments and single implant-supported restorations. Clin Implant Dent Relat Res. 2015;17(3):554-561.
16. Human histology of a CeraRoot implant after 3 months of surgery. CeraRoot. ceraroot.com/professionals/human-histology-of-a-ceraroot-implant-after-3-months-of-surgery/. Accessed April 17, 2018.